MECOM rearrangements are detected in myeloid neoplasms [myelodysplastic neoplasm (MDS) and acute myeloid leukemia (AML)] which are associated with dismal prognosis. Classic MECOM rearrangements include inv(3)(q21q26.2) and t(3;3)(q21;q26) while non-classic subtypes are 3q26.2/ MECOM rearrangement with other partners. Both classic and non-classic rearrangements result in MECOM overexpression which involves in the process of leukemogenesis. Recently, the World Health Organization classification 2022 categorized myeloid neoplasms with these genetic abnormalities as “AML with MECOM rearrangement” regardless of blast count. We aim to explore frequency, clinical characteristics and outcomes including treatment response in this AML subtype among Thai myeloid neoplasms.
AML data was collected from a national registry which were conducted by Thai acute leukemia working group. Other than AML with MECOM rearrangements, AML was categorized as favorable, intermediate, and adverse risk groups according to European Leukemia Network 2022. MDS data was collected from a multicenter study group involving 4 medical centers. Other than MDS with MECOM rearrangements, MDS was categorized into 5 risk groups according to R-IPSS score system (very low, low, intermediate, high and very high-risk groups). Myeloid neoplasms with MECOM rearrangements were analyzed among their diseases and grouped together and compared to MDS and AML cohorts.
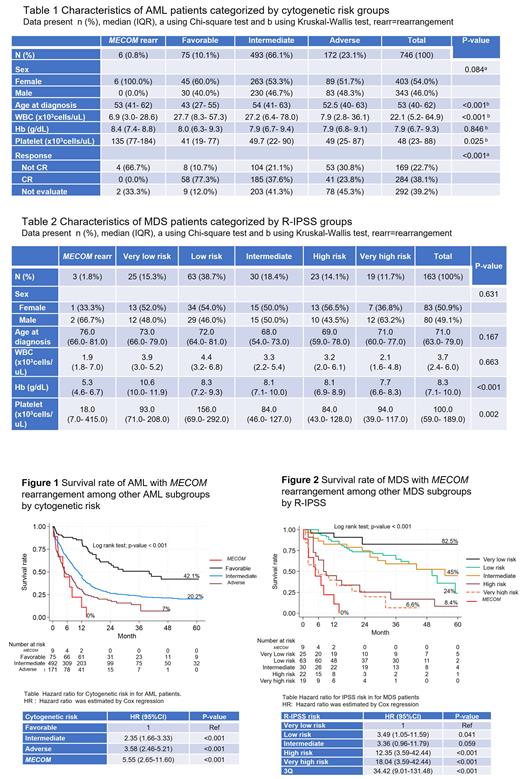
A total of 9 cases of myeloid neoplasms with MECOM rearrangement were detected. Among non-M3 AML cases, there were 6 cases with MECOM rearrangement from 746 non-M3 AML (0.8%) while 3 cases were detected in 163 MDS (1.8%). Seven of 9 cases (78%) were female gender. Five of 9 cases were classic MECOM rearrangement [1 case with inv(3)(q21q26.2) and 4 cases with t(3;3)(q21;q26)] while the other 4 cases were non-classic rearrangements [3 cases with t(3;21)(q26.2;q22) and 1 case with t(3;7)(q26;q21)].
In the AML cohort, AML with MECOM rearrangement showed lower white blood cell, but higher platelet counts compared to other groups (favorable, intermediate, and adverse risk groups) ( Table 1). Among AML cases receiving intensive chemotherapy, MECOM rearrangement subgroup showed lower complete response (CR) rate compared to others favorable, intermediate, and adverse risk groups. (0% vs. 77.3% vs. 37.6% vs. 23.8%; p<0.001). Of note, among 6 AML with MECOM rearrangement, there were 4 patients who received intensive chemotherapy but none of them responded to the treatment.
In the MDS cohort, MDS with MECOM rearrangement showed lower hemoglobin and platelet counts compared to other groups ( Table 2). Among 3 MDS with MECOM rearrangement, one patient received azacitidine with investigational drug (sabatolimab/placebo) and achieved complete hematologic response. He eventually progressed after 12 cycles of the treatments and subsequently died.
When combining 3 MDS and 6 AML with MECOM rearrangement as one group and compared survival rate with others: survival rate of 9 myeloid neoplasms with MECOM rearrangementis worse than the adverse group of AML and the very high risk group MDS with a 1-year survival rate of 22% ( Figure 1 and 2).
In conclusion, myeloid neoplasms with MECOM rearrangements are rare with the frequency of 0.8% in non-M3 AML and 1.8% in MDS. This subtype is more common in female gender. The prognosis of myeloid neoplasms with MECOM rearrangement is dismal with a 1-year survival rate of 16.7% in AML and 6-month survival rate of 33% in MDS. Chemotherapy should be avoided in this subtype due to non-responsiveness, hypomethylating agent showed benefit and can be considered as a bridging treatment before stem cell transplantation. Novel therapy targeting MECOM gene should be further explored to improve outcomes in this AML subtype.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal